Learn more about our latest FDA approved medicine
Product
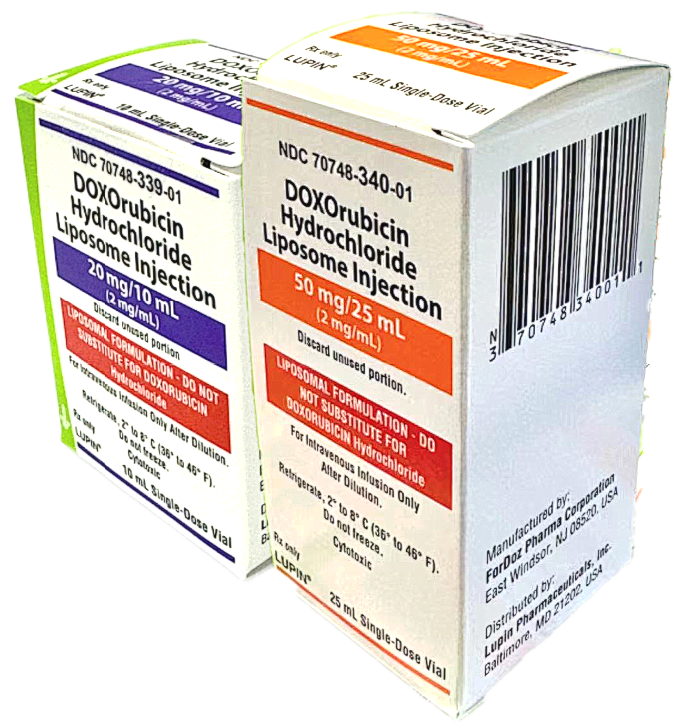
DOXOrubicin Hydrochloride Liposome Injection
20 mg/10mL (2mg/mL) (NDC 70748-339-01)
50 mg/25mL (2 mg/mL) (NDC 70748-340-01)
The FDA approval of our ANDA DOXORUBICIN HCL LIPOSOME INJECTION marks a major milestone for ForDoz as our 1st liposome pharmaceutical product to enter the US market. We are committed to commercially manufacturing this approved liposomal product in our cGMP commercial manufacturing facility at East Windsor, New Jersey.
The product will be marketed in the U.S. through our marketing and sales partner Lupin Pharmaceuticals, Inc. Please read the full prescribing information.